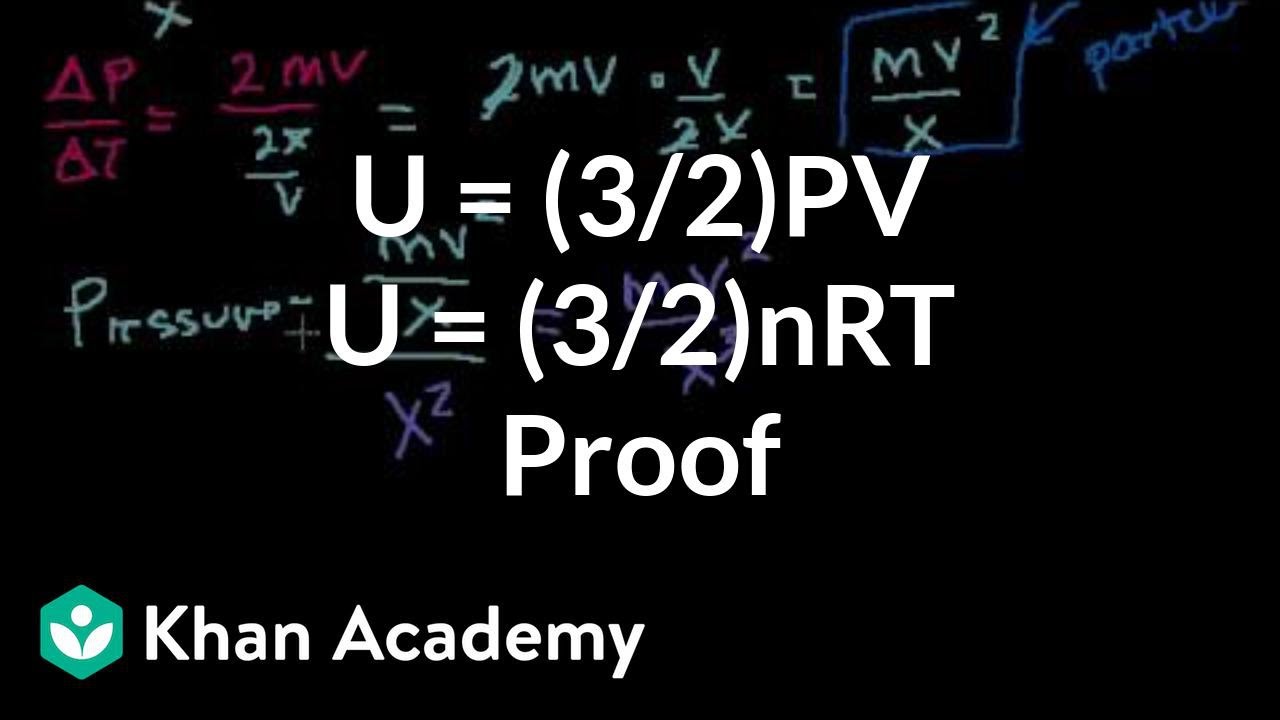
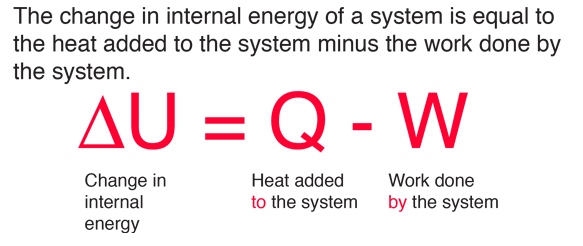
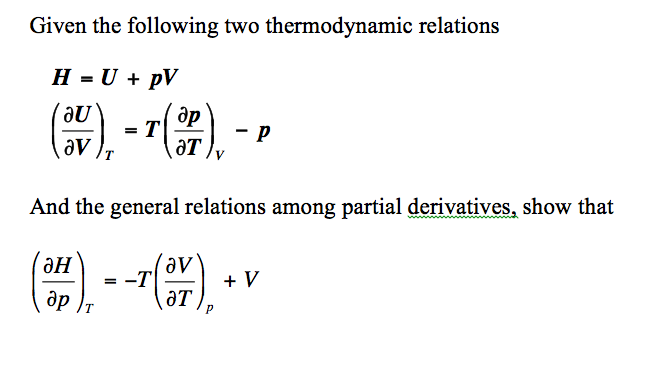
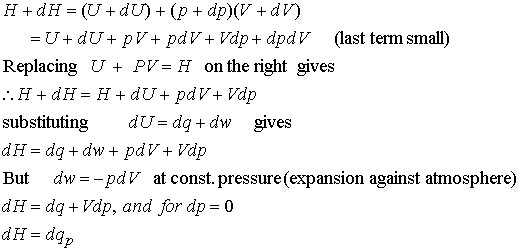The internal energy of an ideal gas is sum of totalkinetic energy of all molecules. Consider an idealgas in which the relation among U, P and V isU =2 +3 PV. The

The internal energy of an ideal diatomic gas corresponding to volume V and pressure P is U = 2.5 PV. The gas expands from 1litre to 2 litre at a constant pressure

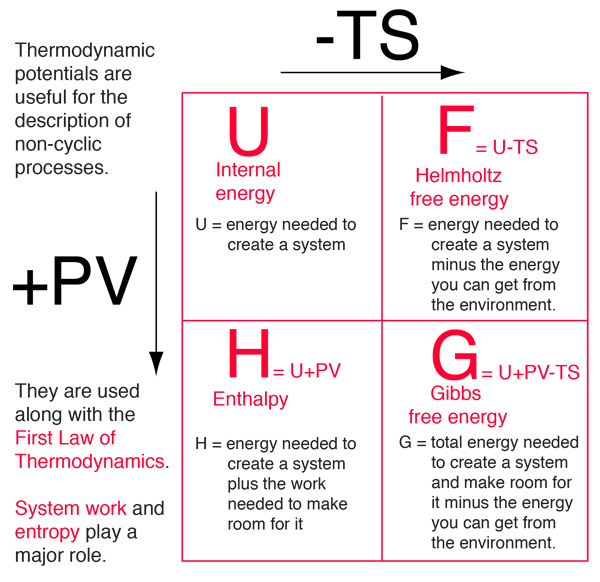
Difference Between Enthalpy and Internal Energy | Definition, Units, Formula for Calculation, Properties, Examples